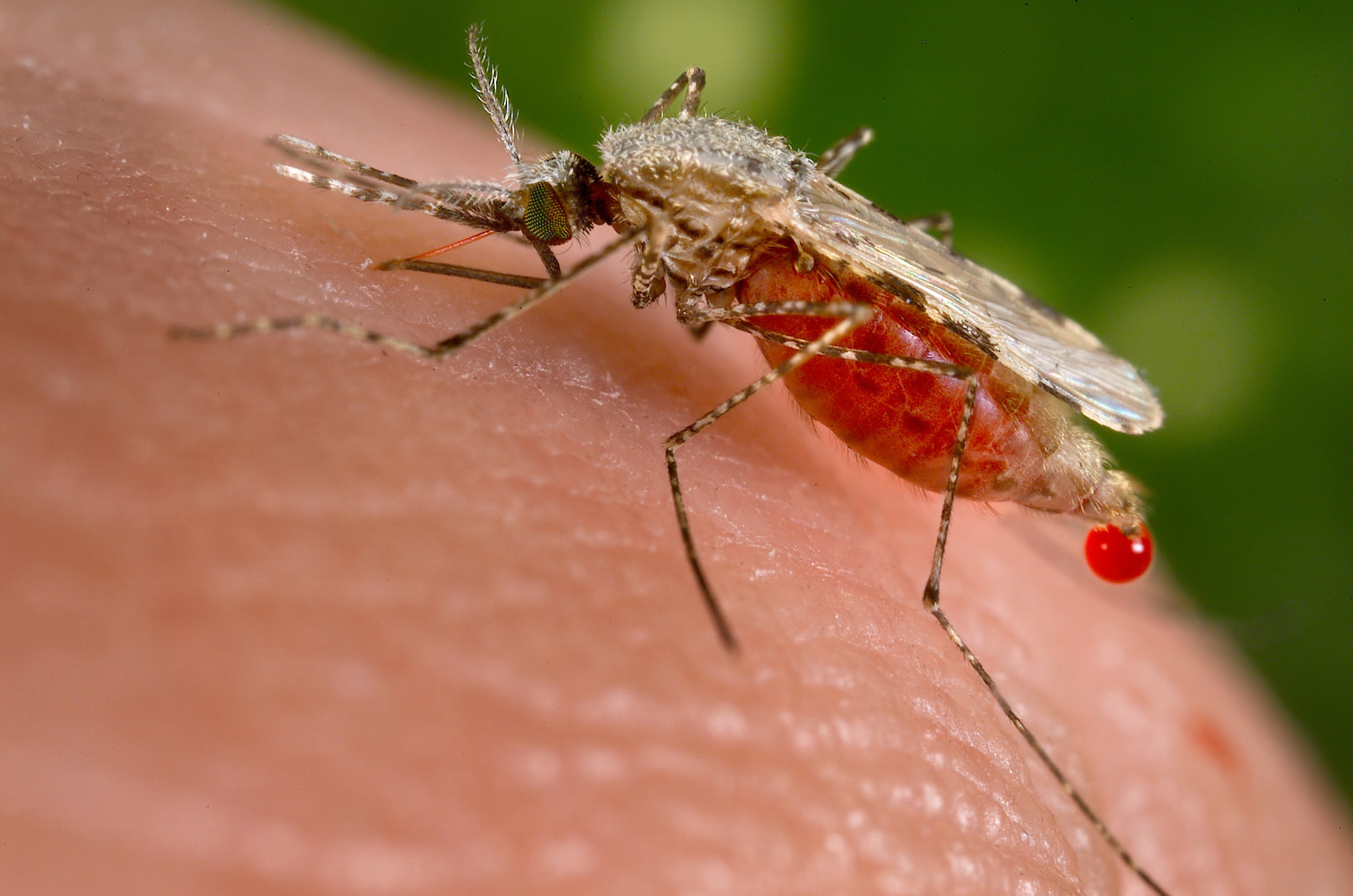
Today is World Malaria Day. It’s a reminder that malaria still kills.
In 2015, there were an estimated 212 million malaria cases, and 429,000 people died. Most of those deaths were women and children; malaria is especially fatal to pregnant women and children under the age of five. To be sure, the world has been making global progress against malaria – malaria incidence among populations at risk fell by one-fifth from 2010 to 2015. However, that progress is slow. In part, that is because Malaria is a very difficult disease to eradicate the complex lifecycle of the malaria parasite and its relationship to mosquitos has vexed scientists for years.
But now, a new vaccine against malaria has the potential to make a massive difference. The vaccine would make the malaria parasite and the mosquitos that spread the disease irrelevant. It would trigger a virtuous cycle, in fact. An effective vaccine would mean fewer people infected with malaria, and when fewer people are infected, mosquito transmission decreases. According to Dr. Matshidiso Moeti, the World Health Organization Regional Director for Africa, “Combined with existing malaria interventions…a vaccine would have the potential to save tens of thousands of lives in Africa.”
As of today, the world is be one big step closer to that vaccine.

Ghana, Kenya, and Malawi will take part in a WHO-coordinated malaria vaccine implementation project that will make the world’s first malaria vaccine available in selected areas beginning in 2018. The vaccine, called RTS,S, was developed to protect young children from the most deadly form of malaria. It will be assessed in the pilot project to see if it as an effective malaria control tool when combined with existing anti-malaria interventions.
RTS,S as already passed phase three vaccine testing. This means we know that it is safe in humans and that it does produce an immune response against malaria in people who have been vaccinated, at least in children ages 5-17 months. We do not know, however, if the vaccine will be effective in real conditions. The pilot project is designed to find out three things. Logistically, is it actually possible to deliver all four doses of vaccine? Can the vaccine reduce the number of deaths in childhood? Is it safe in the context of routine use?
This is the first malaria vaccine to have successfully completed a Phase 3 clinical trial. The trial itself was the result of new financing approaches – it was conducted between 2009 and 2014 through a multifaceted partnership involving GSK, the PATH Malaria Vaccine Initiative, support from the Bill & Melinda Gates Foundation, and a network of African research sites in seven African countries—including Ghana, Kenya, and Malawi.
The three countries were chosen for the vaccine trial because they already have highly functional malaria control programs and good immunization programs while still facing a high burden of malaria. They also have good population coverage with the insecticide treated bednets that prevent mosquito bites. The countries will select target districts and regions for the pilot on their own, based on malaria burden and logistical considerations. Finance for the trial will also come from a partnership – Gavi, the Vaccine Alliance, the Global Fund to Fight AIDS, Tuberculosis and Malaria and UNITAID are all partnering to provide $49.2 million for the first phase of the pilot program. It will be complemented by in-kind contributions from WHO and GSK.
An effective malaria vaccine would save thousands of women and children, and it would serve as a force multiplier of the progress already being made in malaria control. The first phase of the pilot project is just three years long.
By the end of 2020 we’ll know if we have a shot at ending malaria once and for all.